By Al Bennett – April 2021
Introduction
We read and hear a lot about nitrogen and phosphorous as nutrients that significantly impact the aquatic life of our lake. Typically, the challenge in lakes worldwide is to minimize the presence of these nutrients so that algae and cyanobacteria growth is not overly stimulated. This article briefly touches on how nitrogen and nitrogen compounds, found in what is called the nitrogen cycle, are used in our daily lives. The article then explores the nitrogen cycle in a lake like ours (i.e., how nitrogen enters our lake from the atmosphere, is converted into nitrogen compounds, and is released back to the atmosphere). The focus here is on the natural cycle of nitrogen and not how it enters our lake through manmade means (commercial fertilizer, output from waste treatment, faulty septic systems, runoff from dairy, beef, and chicken operations, etc.).
Common Uses
Nitrogen is all around us and is a key to life. Nitrogen makes up approximately 78% of the earth’s atmosphere and it is essential for the making of DNA and proteins. About 3.3% of the human body’s mass is made up of nitrogen. In addition to being a key to life, we use nitrogen daily in varying forms. The below table lists the different forms of nitrogen that occur in the nitrogen cycle and some of their common uses in our daily lives.
|
Element/Compound
|
Some Common Usages
|
|
Nitrogen
|
In liquid form, it is what a dermatologist uses to freeze skin anomalies; used to quickly freeze foods; and used to preserve blood, reproductive cells (sperm and egg), and other biological samples and materials. In gaseous form, it is the gas released to inflate a cars air bag and it is used in the manufacturing of stainless steel to remove impurities.
|
|
Ammonia
|
About 80% produced is used in fertilizer. Also used as a household cleaner, refrigerant gas, and has a multitude of commercial applications.
|
|
Nitrites
|
In the form of sodium nitrite is used as preservative in processed meats. Also gives meat that pink-red fresh look.
|
|
Nitrates
|
Also used as a meat preservative. Used in several medicines including nitroglycerine and is used as an oxidizing agent in explosives.
|
|
Nitrous Oxide
|
Commonly referred to as laughing gas and sometimes used by dentists to sedate patients. Used as the propellant in aerosol whipped cream canisters and cooking sprays. In car racing, provides more oxygen to the engine combustion cycle enabling more fuel to be burned and thereby increases engine horsepower.
|
Nitrogen Cycle
The nitrogen cycle in a lake environment is a continuous cycle of biochemical processes, all occurring simultaneously, by which nitrogen is absorbed at the water’s surface, converted into various chemical forms, and then returned to the atmosphere.
Nitrogen in its natural form is a gas (N2) and like oxygen, nitrogen is soluble in water. Nitrogen can enter the lake through precipitation, but it mostly enters through diffusion at the water’s surface where the concentration of nitrogen in the atmosphere is much higher than the concentration of nitrogen in the water. The diffusion of nitrogen occurs slowly across the water’s surface and can occur more quickly through sources of aeration such as wind created waves. The amount of nitrogen that can be absorbed by water is approximately 20 mg/l at 68 degrees. 
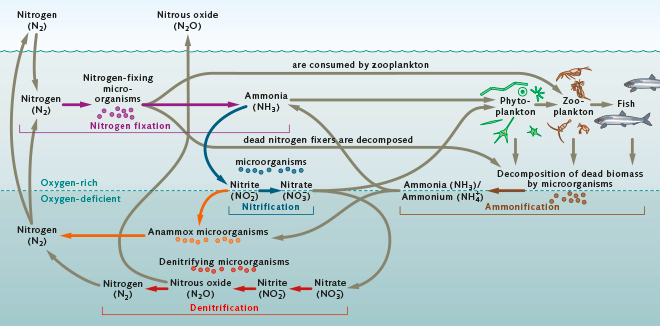
Nitrogen is the most plentiful element in the Earth’s atmosphere as well as an important plant nutrient. In nature, nitrogen circulates and is continuously converted chemically from one form into another by bacteria and plants. The above figure is taken from World Ocean Review.
Pure nitrogen is unusable to most organisms. It must undergo a series of transformations to convert it to a compound that can be absorbed by aquatic plants and eventually consumed by fish. The first step in this process is called nitrogen fixation. It is a bacterial process whereby bacteria, including cyanobacteria, convert the nitrogen dissolved in water into ammonia/ammonium (NH3/NH4+). In a lake like ours where the water’s ph (a measure of how acidic/basic water is)is typically below 8.75, more ammonium than ammonia is produced.
The next step in the overall nitrogen cycle is called nitrification. It is a two step process where bacteria convert the ammonia/ammonium into nitrite ions (NO2-) and different bacteria then convert the nitrite ions into nitrate ions (NO3-).
The nitrogen-based ammonia/ammonium and nitrate ion compounds produced through fixation and nitrification are nutrients to the lake’s food chain and are first taken up into the tissues of algae (phytoplankton) and aquatic plants. Zooplankton (microscopic fish) and some aquatic insects eat the algae which in turn are eaten by small fish. Larger fish may then feed on zooplankton, insects, and/or smaller fish. The incorporation of ammonia/ammonium and nitrate into the biological tissues of aquatic life is commonly referred to as the assimilation step in the nitrogen cycle.
Following assimilation is ammonification. Eventually, the lake’s bio-mass consisting of phytoplankton, zooplankton, fish, and all other forms of aquatic life will die and decompose. In the decaying process, specialized bacteria convert the nitrogen compounds contained within the decaying bio-mass back into ammonia/ammonium.
This ammonia/ammonium again undergoes nitrification similar to the nitrification step above where the ammonia/ammonium is converted back to nitrate ions.
As a last step in the nitrogen cycle, the produced nitrate ions then undergo a denitrification process where denitrifying bacteria convert the nitrate ions back to nitrogen as well as nitrous oxide (N2O). Both are gases that are released back to the atmosphere.
The natural nitrogen cycle in marine and terrestrial environments is very similar to that in fresh water environments. In all cases, nitrogen is extracted from and returned to the atmosphere. If this were not the case, our atmosphere would quickly be depleted of nitrogen.
